Abstract
Background: Generalizability of results from clinical trials with narrow in-/exclusion criteria are a concern as significant deviations in efficacy or toxicity may occur when treatments are used in populations of elderly and more comorbid patients. The cost-effectiveness of novel therapies may also be less favorable if real-world treatment effects are inferior to clinical trial results. In diffuse large B-cell lymphoma (DLBCL), a recent US study showed that baseline organ function-based eligibility criteria had substantial impact on survival with ineligible patients being at higher risk of dying from progressive lymphoma (1).
Aims: The present study explored the impact of commonly used in-/exclusion criteria in key completed and ongoing first line DLBCL trials on survival in a Danish population-based study of patients with de novo DLBCL.
Patients and methods: DLBCL patients enrolled in the Danish Lymphoma Registry (LYFO) and treated with R-CHOP in the period 2008-19 were screened for completeness of data to match trial eligibility criteria. The key organ/hematology eligibility criteria of completed or ongoing all-comers DLBCL studies (REMoDL-B, Goya, Polarix and Hovon84) were collected (bilirubin, ALAT, creatinine, eGFR, leucocytes, neutrophils, thrombocytes and ECOG).
First, high-level trial matching on disease risk group (Ann Arbor, bulky disease, international prognostic index (IPI)) and age-groups were performed so that patients assessed for trial eligibility had a relevant risk profile. Subsequently, patients were divided into eligible or ineligible based on selected in/exclusion criteria. For each trial, overall survival (OS) from treatment start were compared for eligible and ineligible patients using inverse probability of treatment weighted Kaplan-Meier and log-rank tests. Crude OS and OS adjusted for residual imbalances in IPI and age were estimated. When possible, the OS curves from standard arms of the original trials were superimposed on OS plots. For each trial, the Shapley value of each criterion was calculated, using the HRs as well as the 5y restricted loss of lifetimes (RLOLs). The Shapley value measures the average influence of each eligibility criterion on the estimated IPI- and age-adjusted HR/5y RLOL.
Results: A total of 3,150 R-CHOP treated DLBCL patients without discordant low-grade lymphoma were identified in the surveyed period. A total of 1,666 patients (52.89% of surveyed population) were available for the REMoDL-B trial, 1,431 (45.43%) for Goya, 1,125 (35.71%) for Polarix, and 1,432 (45.46%) for Hovon84. The variations of numbers for each trial evaluation were explained by trial inclusion criteria and missing data in LYFO. Crude OS estimates for patients with and without all necessary information were similar (data not shown).
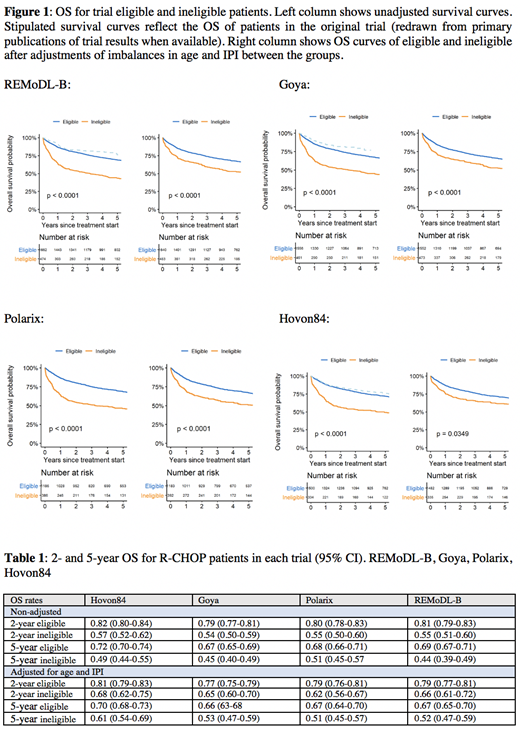
OS curves for eligible and ineligible patients are shown in Figure 1 and, when possible, with superimposed trial results (Goya, REMoDL-B, Hovon84). Survival differences between trial eligible and ineligible patients were robust to further adjustment of imbalances in age and IPI.
Associated crude and adjusted 2 and 5-year OS rates for trial eligible and ineligible are shown in Table 1. The largest numerical difference in 2-year crude OS between eligible and ineligible was observed in the REMoDL-B trial (ineligible had 26% lower 2-year OS rate). The largest numerical difference in 2-year OS adjusted for IPI and age between eligible and ineligible was observed for the Polarix trial (ineligible had 17% lower 2-year OS rate). The strongest drivers of OS differences between trial eligible and ineligible patients in terms of the tested eligibility criteria were thrombocyte count (HR-contribution calculated from Shapley values -0.11; -0.14) and ECOG (HR-contribution -0.09; -0.21). Liver function parameters (bilirubin and ALAT) had low impact on OS (HR-contribution 0.00; -0.05 and 0.00; 0.07).
Conclusions: The present population-based study confirms that trial ineligible patients have worse survival even after adjustments in imbalances in age and disease risk category. Thus, trial eligibility criteria have substantial impact on generalizability of results to a wider unselected population. Interestingly, the trial eligible patients identified in the present study had very similar outcomes to R-CHOP treated patients in the original trials supporting the possible use of RWD as synthetic control arms.
Maurer: Kite Pharma: Membership on an entity's Board of Directors or advisory committees; Morphosys: Membership on an entity's Board of Directors or advisory committees, Research Funding; Nanostring: Research Funding; Genentech: Research Funding; Celgene: Research Funding; Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees. Jørgensen: Novartis: Consultancy; Gilead: Consultancy; Roche: Consultancy; Celgene: Consultancy. Larsen: BMS: Consultancy; Novartis: Consultancy; Celgene: Consultancy; Odense University Hospital, Denmark: Current Employment; Gilead: Consultancy. Clausen: Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel expences ASH 2019; Gilead: Consultancy, Other: Travel expences 15th ICML ; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees. Poulsen: Abbvie: Consultancy; Janssen: Consultancy. El-Galaly: Abbvie: Other: Speakers fee; ROCHE Ltd: Ended employment in the past 24 months.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal